Monitoring
What You Need to Know About Environmental Monitoring in Life Sciences
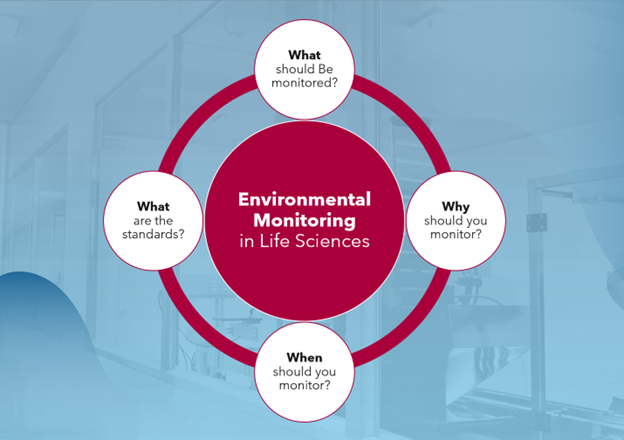
Environmental monitoring in life sciences is essential for ensuring product safety, regulatory compliance, and overall operational integrity.
Environmental monitoring in the pharmaceutical and biotech industry is all about safety – asset safety, product safety, patient safety, staff safety, and compliance safety. It involves safeguarding your products by closely monitoring the conditions of:
- Freezers and controlled temperature units.
- Laboratories and cleanrooms.
- Warehouses and storage.
- Production facilities.




