Depending on the size of your chamber, the number of sensors and their placements will vary. Here’s a simple guide to get you started:
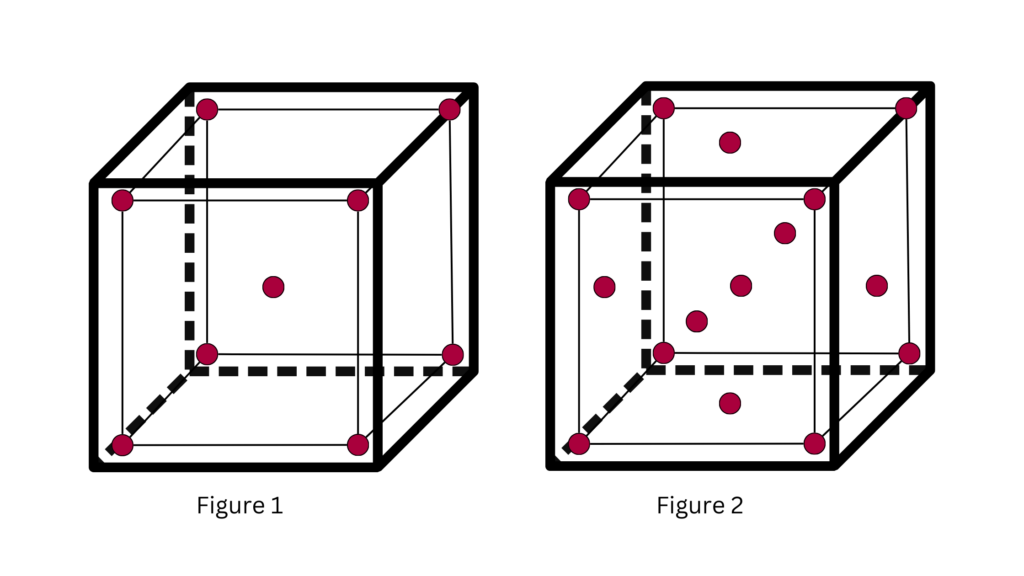
- For Chambers Up to 2 Cubic Meters: Aim for 10 temperature sensors, with nine placed within the chamber to capture a detailed temperature profile and one near the monitoring/control probe for correlation. As illustrated in Figure 1.
- For Chambers Up to 20 Cubic Meters: This size requires 16 sensors. Fifteen should cover the chamber’s interior, while an additional sensor near the monitoring/control probe ensures data consistency. Align with the configuration shown in Figure 2.
- For Larger Facilities: The approach changes slightly. Here, it’s about creating a grid, with sensors spaced every 5 to 10 meters, and adjusting based on the facility’s specific risks and characteristics. For exceptionally large spaces, consider placing sensors up to 20 or 30 meters apart, based on a risk assessment that considers the unique environmental characteristics and the products stored. Don’t forget to monitor the ambient temperature, as external factors can significantly impact the internal environment.

These recommendations come from a deep dive into guidelines provided by reputable sources like ISPE, USP, and WHO. They’re not just numbers pulled out of thin air; they’re backed by years of research and practical application.
🌐 ISPE (2021). Good Practice Guide: Controlled Temperature Chamber Mapping and Monitoring.
🌐 USP (2018). USP41-NF36 <1079>. Good Storage and Distribution Practices for Drug Products.
🌐 World Health Organization (2015). Technical Supplement 8 to WHO Technical Report Series, No. 961, 2011. Temperature Mapping of Storage Areas.
These guidelines should serve as a foundation and are adaptable to your facility’s needs, considering unique environmental characteristics for accurate mapping and resource optimization while maintaining product integrity and safety.