Validation
Annex 1 Evolution: Forging the Future of Pharma Safety with Ellab

Explore how Ellab leads pharma safety with innovative solutions aligned with GMP Annex 1 guidelines for risk and quality management.
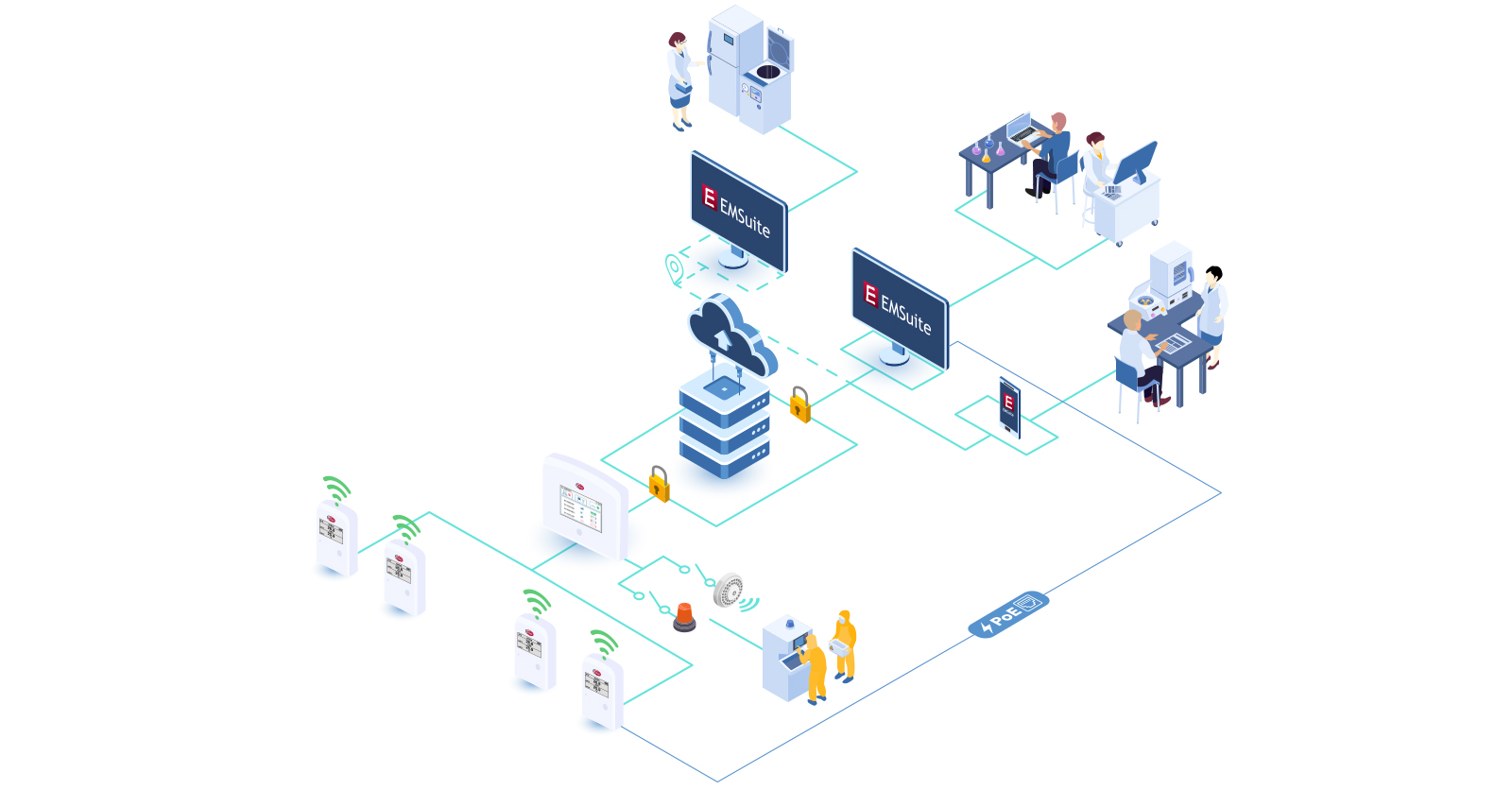
The European Commission’s Good Manufacturing Practice (GMP) guidelines, specifically the refreshed Annex 1, mark a transformative moment for the manufacture of sterile medicinal products. This significant update, reflecting a harmonization of technological progression, methodologies, and an advanced understanding of best practices, ushers in a new chapter in pharmaceutical manufacturing. The revisions resonate deeply with the industry’s commitment to quality risk management (QRM) principles, setting a precedent for minimizing contamination risks and maintaining the integrity of medicinal products throughout their lifecycle.